About Chinese authority NMPA, Chinese regulation and Chinese market of medical device (IVD)
Tags:
Chinese authority, NMPA, medical device,
In vitro diagnostic reagent (IVD),
CMDE, legislative of medical device, State council order No. 739 in 2021 (former order 680), Product-specific guidance, Chinese standards (GB, GB/T, YY and YY/T),
GB 9706.1-2020,
YY 9706.102-2021,
definition of medical device, Marketing authorization holder (=registrant=legal manufacturers), NMPA trend, adverse event reporting
1. Chinese Authority of Medical Device and IVD
In 2018 National Medical Products Administration (NMPA, Chinese:国家药品监督管理局) replaced former China food and drug administration (CFDA). As one part of State Administration for Market Regulation (SAMR) the NMPA is in charge of supervision of drug, medical device and cosmetics in China. The core task of NMPA is to draft legislative of drug, medical device and cosmetics and to supervise the implementation of the regulations. Besides it, NMPA is also responsible of standards, quality management, registration and post market activities including inspection of abovementioned products.
*In-vitro Diagnostics Regulation belongs also to regim of NMPA
Responsibility of NMPA
(1) To supervise the safety of drugs (including traditional Chinese medicines (TCMs) and ethno-medicines, the same below), medical devices and cosmetics; to draw up regulatory policy plans, organize the drafting of laws and regulations, formulate normative documents, and supervise the implementation thereof; to research and formulate regulatory and supportive policies that encourage new technologies and new products for drugs, medical devices and cosmetics.
(2) To undertake standards management for drugs, medical devices and cosmetics; to organize the formulation and publication of the Chinese Pharmacopoeia and other drug and medical device standards, organize the drafting of cosmetic standards, organize the formulation of the classification management system, and supervise the implementation thereof; to participate in formulating the National Essential Medicine List, and assist in the implementation of the national essential medicine system.
(3) To regulate the registration of drugs, medical devices and cosmetics; to develop the registration system, conduct strict review and approval for marketing, improve measures to facilitate the review and approval process, and organize the implementation thereof.
(4) To undertake quality management for drugs, medical devices and cosmetics; to develop Good Laboratory Practices (GLP) and Good Clinical Practices (GCP), and supervise the implementation thereof; to develop Good Manufacturing Practices (GMP) and supervise the implementation thereof in line with NMPA's responsibilities; to develop good practices on the distribution and use of medical products and guide the implementation thereof.
(5) To undertake post-market risk management for drugs, medical devices and cosmetics; to organize the monitoring, evaluation, and handling of adverse drug reactions, medical device adverse events, and cosmetic adverse reactions; to undertake emergency response management for drugs, medical devices and cosmetics in accordance with law.
(6) To undertake management of qualifications for licensed pharmacists; to formulate regulations of qualifications for licensed pharmacists, and guide and supervise the registration of licensed pharmacists.
(7) To organize and guide the supervision and inspection of drugs, medical devices and cosmetics; to develop the inspection system, investigate and punish illegal activities during the registration process for drugs, medical devices and cosmetics in accordance with law, and organize and guide the investigation and punishment of illegal activities during the manufacturing process in line with NMPA's responsibilities.
(8) To engage in international exchange and cooperation in the regulation of drugs, medical devices and cosmetics, and participate in developing relevant international regulatory rules and standards.
(9) To guide the work of drug regulatory departments of all provinces, autonomous regions, and municipalities directly under the Central Government.
(10) To complete other tasks assigned by the CPC Central Committee and the State Council.
Among centers of NMPA, Chinese medical device evaluation (CMDE) performs technical evaluation of medical device. Its role is quite similar to Center for Devices and Radiological Health (CDRH) at FDA in USA.
Responsibility of CMDE
(1) Be responsible for the acceptance and technical review of registration application of domestic Class III medical device products and imported medical device products; be responsible for the filing of imported Class I medical device products.
(2) Participate in the drafting of relevant laws, regulations and normative documents related to the registration administration of medical devices. Organize the formulation and implementation of relevant technical reviews norms and technical guidelines for medical devices.
(3) Undertake the technical review of medical devices involved in emerging medical products such as regenerative medicine and tissue engineering.
(4) Coordinate the inspection work related to the evaluation of medical devices review.
(5) Carry out researches on the theories, technologies, development trends and legal issues related to medical device review.
(6) Be responsible for providing guidance and technical support for local departments in the technical review of medical devices.
(7) Organize relevant consulting services and academic exchange, and carry out international (regional) exchange and cooperation related to medical device review.
(8) Undertake other tasks assigned by NMPA.
CMDE is quasi the contacting authority during the approval process with whom the overseas manufacturers communicate. CMDE initials also product-specific guidance und participates in inspections.
English Regulation
There is only one registration workflow at CMDE website and a brief English website of NMPA.
2. Chinese Regulations of Medical Device and IVD
The legislative of medical device is developing in rapid tempo in China. The Regulations on Supervisory Management of Medical Devices have been revised and approved at the the 119th Executive Meeting of the State Council end of 2020 and is valid on 01.06.2021. Since then diverse provisions, notifications and technical guidances are published which should be comply with and followed in the whole life cycle of medical device marked in China.
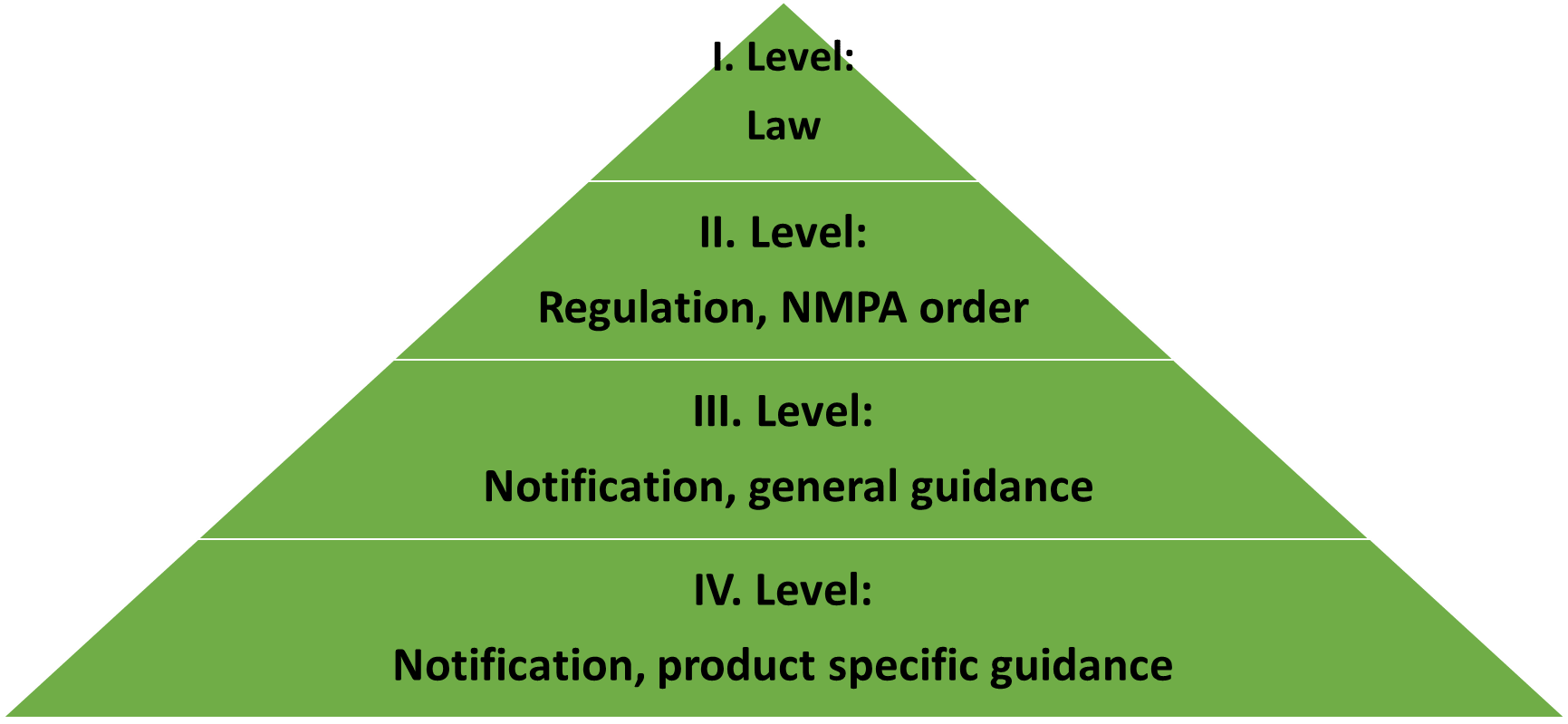
How does legislative of medical device in China build up?
I. Level: Law
“Regulations on supervision and administration of Medical Device” (State Council Order No. 739) has the similar legally role as 2017/745 (Medical Device Regulation, MDR) in EU or FD&C Act in US. State Council Order No. 739 is extremely compactly summarized (20k Chinese characters and 107 articles in No. 739 compared to 100k English words and 123 articles in EU MDR) and has only 8 chapters containing registration, manufacturing, after approval sales, adverse event and recall, after approval supervision as inspections and legal liability of medical device.
New
Medical device act (stand of 2024:Draft) will replace State Council Order No. 739 has firstly the law character...
II. Level: Regulation, NMPA Order
There are many supportive general orders (or provisions) clarifing requirements of product registration, instruction for use, product classification, adverse event and overseas inspection of medical device.
Medical Device Registration and Filing Management Measures (order 47, 2021)
IVD Registration and Filing Management Measures (order 48, 2021)
Guidance for Medical Device Production Quality Management (order. 64, 2014), Chinese GMP
Medical Device Production Supervision and Administration Measures (order 53, 2022)
Guidance for Inspection of Quality Management System for Medical Device Registration (order 50, 2022)
Instruction for use and labels of Medical Device (Order 6, 2014)
Adverse Events Monitoring and Reevaluation of Medical Device (Order 1, 2018)
Guidance for compiling annual self-examination report of medical device quality management system (order 13, 2022)
Overseas inspection of Drug and Medical Device (Order 101, 2018)
III. Level: Notification, general guidance
Actually theses guidance issued by CMDE are often useful guideline of product registration.
Clinical evaluation of Medical Device (Order 73, 2021)
Clinical evaluation of IVD (Order 74, 2021)
Approval of overseas clinical trial data of medical device (Order 13, 2018)
Approval of overseas clinical trial data of In vitro diagnostic reagent (Order 95, 2021)
Technical review of medical software (Order 9, 2022)
Technical review of cybersecurity safety of Medical Device (Order 7, 2022)
Product technical requirement (PTR) of Medical Device (Order 9, 2014)
IV. Level: Notification, product-specific guidance
To discriminate to general guidance, there are product-specific guidance matching most product code.
If you have the first 4 digits of product code or general group of interested product, you can search the corresponding product-specific guidance and standards.
3. Chinese Standards of Medical Device and IVD
national (GB) - and industrial (YY) standards
There are two kinds of Chinese standards:
national (GB) issued by the Standardization Administration of China (SAC), and
industrial (YY)
issued by NMPA. The
non-binding or recommended character has prefix /T. Without /T it means
obligatory. In general the version of Chinese standards is at developing stage. Most Chinese standards match the oldversion or edition of harmonised international standards. There are three categories compared to international
standards: identical (IDT), equivalent (EQV), or non-equivalent (NEQ).
Example
NEW GB 9706.1-2020 (valid as of May, 2026, replacing GB 9706.1-2007)
Corresponding to IEC 60601-1:2012, edition 3, Medical electrical equipment—Part 1: General requirements for safety
NEW YY 9706.102-2021 (valid as of May, 2026, replacing YY 0505-2012)
Corresponding to IEC 60601-1-2:2007, edition 3, Medical electrical equipment-Part 1-2: General requirements for safety-Collateral standards: Electromagnetic compatibility-Requirements and tests
New GB/T 16886.1-2022 (valid as of May, 2023)
Corresponding to ISO 10993-01: 2018, Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process
YY/T 0316-2016
Corresponding to ISO 14971:2007, Application of risk management to medical devices
GB/T 25000.51-2016 Systems and software engineering―Systems and software Quality Requirements and Evaluation (SQuaRE)―Part 51:Requirements for quality of Ready to Use Software Product(RUSP) and instructions for testing
Every year NMPA makes a plan of revision or new medical device industrial standards. This means that the applied products must be compliant with new standards in all three
types of product registration (initial -, change - and extension registration).
If new obligatory national or industrial standard applies to medical device, very often at least a delta of type testing must be undertaken to fulfill the new requirements. It is similar to an international revision of medical electrical standards as IEC 60601 series for every manufacturers.
Likewise the GB 9706.1-2020 will be prolongated in 2026. As of May, 2026 all medical electrical equipments have to fulfil the new safety requirements at testing and then to be able placed in market. Finally one of the important Chinese standards reaches the international stand of the art.
4. Definition of Medical device in China
Definition
Regulations on the Supervision and Administration of Medical Device (State council order 2021/739)
Article 103
Medical device is an instrument, equipment, appliance, in vitro diagnostic reagent and its calibrator, material, and other similar or relevant articles including necessary computer software, directly or indirectly contacting human body; the effectiveness are obtained mainly through physical means other than pharmacological, immunological or metabolic ways, or such ways are involved in but only play auxiliary roles; which is used to achieve the following intended outcomes:
- Diagnosis, prevention, monitoring, treatment or alleviation of diseases
- Diagnosis, monitoring, treatment, alleviation or functional compensation for an injury
- Examination, substitution, regulation or support of physiological structure or physiological process
- Life support or life sustaining
- Contraception Control
- Examination of the sample from human body to provide information for medical or diagnostic purpose
Medical device registrant and record holder
Article 34
can produce medical devices on their own, or they can entrust enterprises that meet the requirements of these regulations and have corresponding conditions to produce medical devices.
Article 103
refer to the enterprise or research institution that has obtained the medical device registration certificate or handled the record of medical device.
Medical device registrant and record holder refer to marketing authorization holder (MAH) of class I, II and II medical device. It is the same as legal manufacturer which means that MAH has full power of attorney for medical device.
5. Trend of registration at NMPA (2022)
New registered products
Initial registration are 2500 (+46.2% compared to 2021), change registrations 4224 (+58.5% compared to 2021) and renewals 5281 (-24.8% compared to 2021)
Approved domestic class III medical devices are 5425 and IVD 1287.
Approved imported class III medical devices are 2377 and IVD 302.
Approved domestic class II medical devices are 1590 and IVD 877.
Approved domestic class II medical device are 32889!
Top 5 foreign countries and top product categories
The top 5 foreign countries are US (226+), Germany (117-), Japan (69-), Korea (42+) and France (26, new) which cover 76.4% of total imported medical device and IVD.
Top 5 product categories (total 22 product categories) of domestic medical device:
13 Passive Implantable Devices (400, +68.4%)
03 Neurological and Cardiovascular Surgical Devices (246, +92.2%)
14 Injection, Nursing and Protection Devices (168, +6.3%)
06 Medical Imaging Devices (162, +78%)
01 Active Surgical Devices (113, +76.6%)
Top 5 product groups (total 22 product categories) of imported medical device:
06 Medical Imaging Devices (75, +7%)
13 Passive Implantable Devices (53, +10.4%)
14 Injection, Nursing and Protection Devices (50, new)
17 Dental instruments (45, +9.7%)
03 Neurological and Cardiovascular Surgical Devices (43, +19.4%)
The increase of registrations above is compared to 2021. The reduction of imported medical device is distinct.
In the top 5 product groups from the review of registered number it is not noteworthy about domination of imported product.
Among the common top product groups, 13 Passive Implantable Devices, 03 Neurological and Cardiovascular Surgical Devices, 14 Injection, Nursing and Protection Devices and 06 Medical Imaging Devices have hunge domestic domination.
What can we support
The regulation of medical device in China is foundation of not only registration of product but also from product design stage.
Besides receiving newsletter (form), you can book a customer tailored regulation monitoring of wished product types and market trend.
Do you need Chinese standard with analysis with international standard? Contact us, we offer all needed translated Chinese standard in English too.
Do you know that one finding of overseas inspection is missing of qualification of responsible person with Chinese regulation?
Manufacturer should have a match of Chinese regulation with corresponding manager and employees. Even at job description it must state the exact Chinese regulation.
Either you are startups, SME of global player, we can offer any kinds of trainings dedicated to medical device.
Contact us for a free consulting to scan you gap, we will offer dedicated workshops onsite or remote.