Outsourced testing, valid for NMPA, MDR in EU and global markets
Tags: outsourced testing, verification and validation, clinical trial with multicentres, medical device, CNAS, ISO 17025, ASCA-Accredited, tetsing in China, 器械检验,欧洲临床检验,CE证书

Background of Chinese testing
Do you have headache of Chinese testing of medical device or IVD or medical components or wireless? It goes always unplanned difficult due to unprofessional support, communication, monitoring. It wastes money and time which is sometime even higher and longer than at registration.
Well known is type testing in China which is an important part at Chinese registration. You have to send your product to a certified testing labs or institutes. Actually you need in some cases more labs to cover different microbiological, chemical, environmental testings.
Unknown is that there are more and more certified Chinese labs and institutes (CNAS, ISO 17025, ISO 9001 and ISO 13485). You can have a quick schedule with applied international standards. With the English report you can serve in different markets (US, EU, Asia, Latin America). So don’t limit your satisfaction for a testing of finished medical device. As medical components, wireless parts and packagings, you can always find cost-saving, qualitative Chinese labs during the development or in pre-market stage, better not briefly prior tosubmission.
Benefit of outsourced testing in China
It is worthy to outsource the Chinese testing. You don’t need strain own R&D department or project management. We have a tool of proved certified Chinese testing labs and institutes. Even not, we can have a research with your need to find wished labs and inspect any new partners upon your QM-requirements.
Here are some benefits to outsource Chinese testings:
· Unburden own R&D
· Compliant to either Chinese or international standards or both
· A tool of certified Chinese labs and institutes
· Project management with testing knowledge
· Optimised SOP of Chinese testing
· Bilingual communication in and outside China
· Accompany the local testing and troubleshooting
· Overseas training to know your product
· Fast and cost-saving project
· Support of any follow up actions with distributor or Chinese agent or authority
· Leverage global registrations by checking final report
Service and more
We list the basic standards upon which the test will be executed. Ask us to which international standards you want to test, we will match the testing labs and institutes.
- GB/T 14170
- IEC 62133, UN38.3
- ROHS, Reach
- GB/T 25000.51
- GB 16886 series (corresponding ISO 10993 series)
- GB 9706 series (valid since May, 2023) corresponding IEC 60691 series
- SRRC, NCC, FCC, MIC testing
- Accelerated Aging, ASTM F1980 & Real-Time Aging
- Your outsourced verification test without applied standards
Contact us, even if you want to
· outsource any Chinese projects as Chinese clinical study
· have tests in any of our international partner labs
· have gap analysis of testing in China of new medical device
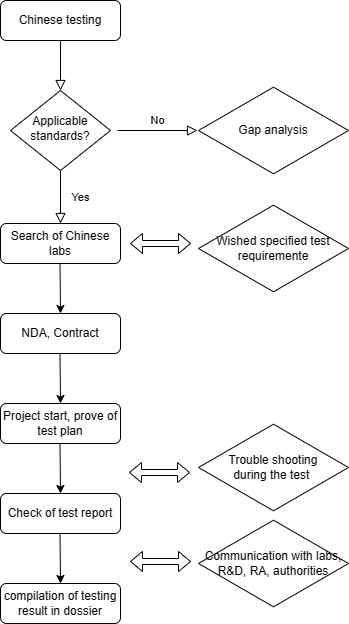
Workflow of outsourced testing of medical device
Below is a basic workflow. In our offer we will concretise the responsibility, time, cost and milestone meeting of testing.
Testing in Europe and worldwide
医疗器械的出海检验
The testing inEurope in many certified labs (certified to ISO 17025 or Certification Body Testing Laboratory or Accreditation Scheme for Conformity Assessment at FDA) can be used for Chinese and global registration. It makes sense to have a testing project beyond the registration preparation because it can be executed parallel and normally takes longer than expected.
We can support you to do testing in Europe too because we have many partner labs who have sound reports accepted by different authority.
Recently the quality and truthfulness of Chinese lab is decreasing expecially at FDA. After warning it makes sence for Chinese manufacturer to assign testing of medical device in Europe.
We can
· accompany from test plan to final test report review
· edition of test summary addressing country regulatory requirements
· answering deficiency reply (or additional request) by different internal agencies and notified body
· have life cycle control when to repeat testing or revise the testing summary
General testing
- IEC 80601 series
- Safty ad EMC and IEC 60601 series
- IEC 61326 series
- ISO 11135, ISO 11137, ISO 17665
- Chemical composition
- Transport ISTA 3A or ASTM D4169
- Surface analysis (XPS, SEM, EDX)
- Validation of process (sterilization, disinfection, clean room, freeze drying, chemical synthesis and decontamination)
Biological evaluations
· Biocompatibility (ISO 10993 – and ISO 18562 series)
· Toxicology
· Mutagenicity
· Genotoxicity
Some special testings for FFP masks, disinfectants, compression textiles and inhalation devices
Cleaning validation of health care products—Requirements for development and validation of a cleaning process for medical devices.
Assessing Credibility of Computational Modeling through Verification and Validation: Application to Medical Devices
Customized laboratory testing
Depending on device or IVD or component you need special testing aligning on not typical standard. We can develop testing method to identify the questions
Clinical study according to ISO 14155:2020
it can be multi-centre international clinical study of medical device. The advantage of clinical trial in Europe is real data from european patients.





