Session 2.4 Clinical evaluation and clinical study in China
Chinese Clinical Evaluation
Just a little different with some Chinese requirements
Clinical evaluation and clinical study is an essential part of submission dossier at medical device registration at NMPA in China. The principle of clinical study is to enable the intended use of medical device, to accept the product risk due to outweighing benefit and to prove conformity of safety and effectiveness requirements.
The philosophy of clinical evaluation in China is based on substantial equivalence at FDA, though there is no requirement of clinical evaluation in USA. The regulation of Chinese CER (new in 2021) aligns almost with European regulation besides the post market details. The subject device will be compared to equivalent or similar devices, which normally has the same product group (Chinese product code), similar intended use and technical characteristics.
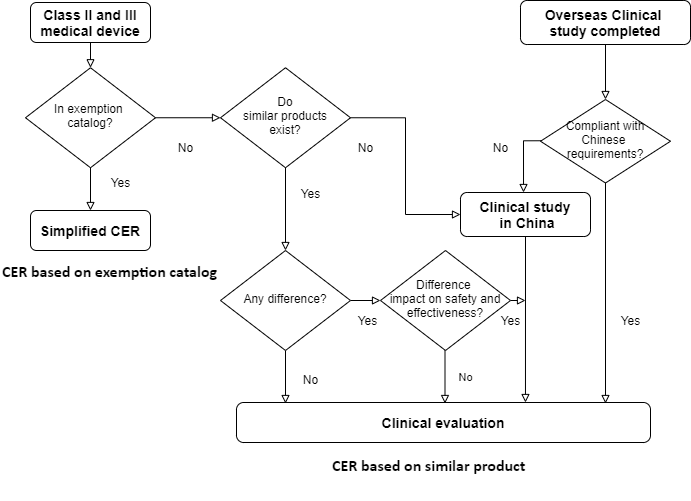
There are 4 alternatives of clinical evaluation to prove conformity of safety and effectiveness requirements of medical device in China:
- CER based on exemption catalog
- CER based on equivalent or similar devices
- CER with accepted overseas clinical study
- Clinical study in China
We would assess the requirements of clinical evaluation of class II and III medical device in China.
In real project we will provide a template of clinical evaluation in details.
1. CER based on exemption catalog
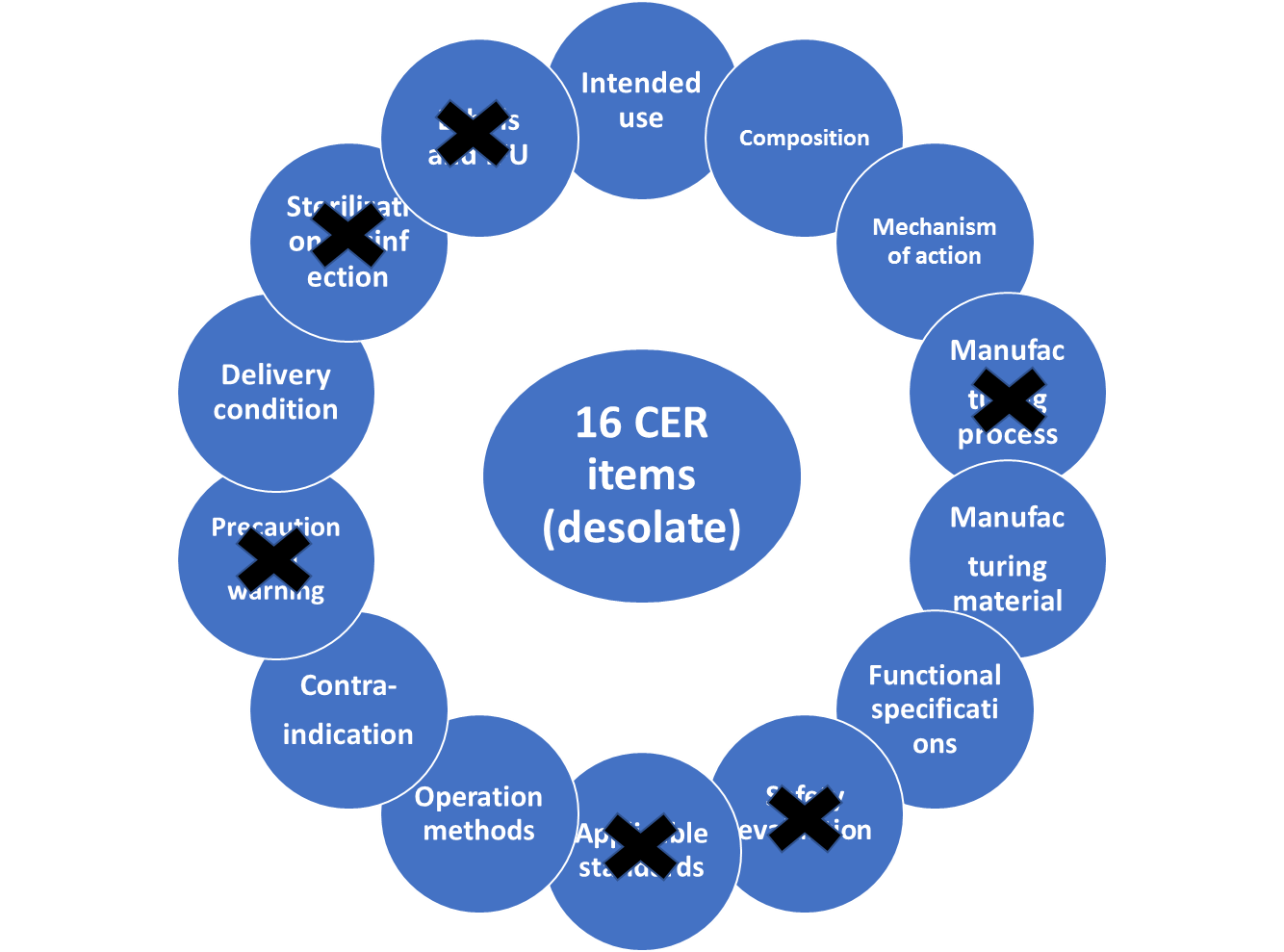
If the product is in “catalogue of medical device exempted from clinical trials”, the manufacturers need compare subject device to the description in catalogue and to items as intended use, composition, mechanism of action, manufacturing materials, functional specifications, sterilization/disinfection and operation method of equivalent devices which are proved in China. This is main part in the simplified clinical evaluation on exemption catalog.
CER based on exemption catalog is excluded if the subject medical device compared to similar medical device has:
- New material
- New technology
- New design
- New active ingredients
- New mechanism of action
- Change or expand of intended use
- Performance specifications not conform with Chinese standards
Update of "catalogue of medical device exempted from clinical trials"
So far (Oct, 2021) there are 1010 product codes listed on “catalogue of medical device exempted from clinical trials”. For the relevant medical device, manufacturers need just a simplified CER.
2. CER based on equivalent or similar devices
If the product is not in the catalogue, however equivalent devices (either own former medical device or medical device from other manufacturers) approved exists in China, a decent clinical evaluation compared to equivalent devices is required.
The scope of this kind of clinical evaluation should conform to new guidance “Clinical evaluation of Medical Device, No. 73, 2021” / Clinical evaluation of IVD, No. 74, 2021 and it is quite similar to requirements at 2017/745 MDR (annex XIV and XV) and Revision 4 of the MEDDEV 2.7/1 at MDD in Europe.
Template of Chinese CER
We introduce the main chapters (New in 2021) in Chinese clinical evaluation:
- Product description and R&D background
- Scope of clinical evaluation
- Clinical evaluation path
- Analyze and evaluate the clinical data with equivalent devices
a. Perform clinical evaluation through clinical data of equivalent devices
- Equivalence assessment
- Summary and evaluation of clinical data of equivalent devices
- Analysis of clinical data of equivalent devices
b. Analyze and evaluate through clinical trial data
5.Conclusion
6.Personal of clinical evaluation
Definition
Clinical data is information concerning safety or performance that is generated from the use of a device.
Clinical data = pre-clinical study + clinical literature + clinical study + adverse events + corrective actions etc.
Equivalent devices
To determine equivalent devices, the following items should be compared between subject - and equivalent devices.
As in figure 3 to see, the scope of application, technical - and biological characteristics are main criteria to select equivalent devices at Chinese clinical evaluation. It is almost the same as at Clinical Evaluation – Equivalence (MDCG 2020-5) at which the scope of application is called clinical characteristics.
Equivalent devices ≠ similar devices
In the new guidance a definition of similar devices is introduced. There might be difference between subject – and to compared devices. The difference will be analyzed in term of three categories in figure 3. In this case the supporting material can be laboratory (pre-clinical) testing and data from clinical trial. If supporting materials include laboratory testing, the testing plan and test report should be submitted as attachments.
3.CER with accepted overseas clinical study
Can overseas clinical study of medical study accepted by Chinese authority? The answer is definitely yes upon Chinese conditions. We help you to analyze the guidance “Admission of overseas clinical trial data of Medical Device” in 2018. Prerequisite is an overseas clinical study in accordance to ISO 14155 in good clinical practice. The overseas manufacturers must provide at least clinical study plan, ethical opinion and clinical study protocol.
The factors below must be considered by accepting overseas clinical study:
- Endpoint of clinical study
- Deviation of population (Asian ethnic)
- Study condition is essential
Manufacturer could explain rational why Asian ethnic doesn’t apply to subject project and make a conclusion that supplementary clinical study in China is not required.
There is good trend that NMPA try to accept more and more overseas clinical study while aligning with international regulation of medical device. It is recommended to check the clinical requirements in China in prior to start oversea clinical study and even Post-Market Clinical Follow-up (PMCF) study (EU). It is worth considering Chinese clinical requirements in a multi-center clinical study.
4. Clinical study in China
The most complex and cost intensive scenario is that the overseas manufacturers have to conduct clinical study in China. It applies to some class III medical devices which are as high risk products listed by authority although the overseas clinical study could be accepted. The clinical study is most likely for innovative products without similar products approved in China. Normally at least 2 clinical research organizations are needed for clinical study in China.
This is a rough workflow of clinical study in China. The input is clinical study plan. The first application is at ethics committee. Followed by application of clinical study at authority, if there is no feedback from authority in 60 workings days manufacturer can conduct the clinical study at hospital. At the end the study protocol is integrated into clinical evaluation as clarified above.
In prior to application of clinical study the following documents are to submit at ethics committee:
- Clinical study plan
- Researcher's manual
- Informed consent
- Procedural documents for recruitment and promotion of subjects
- Case report form
- Self-inspection report and type testing report
- Researcher's resume, professional expertise, ability, training and other documents to prove their qualification
- Facilities and conditions of clinical study organization
- A statement that the development of the test medical device complies with the relevant requirements of the applicable medical device quality management system
Subsequently an application of clinical study with documents below takes place:
- Application form
- Country of origin approval certificate, ISO 13485 certificate, free sales certificate
- Device description
- Research information: preclinical tests, adverse event report, clinical benefit to risk report
- Product technical requirement
- Type testing
- Instruction for use and label
- Clinical study plan
- Agreement with ethics committee
The official cost is
5,3k Euro for application for clinical study and the technical review takes around 60 workings days.
Outlook of clinical evaluation in China (2021)
45% of total medical device groups are exempted from clinical evaluation. 42, 5% of medical device need clinical evaluation whereas only 12, 5 % of total medical device have to undergo clinical study.
Pathways of CER for 7 product groups explained by Chinese authority
In May, 2022, CMDE issues a notification of pathways of clinical evaluation for product groups. Depending on the best practice on the market, Chinese authority makes clear in the 7 lists, for each product code which CER-pathway should be selected.
The listed 7 product groups:
· 11 Disinfection and Sterilization Devices of Medical Devices
· 12 Active Implantable Devices
· 13 Passive Implantable Devices
· 14 Injection, Nursing and Protection Devices
· 15 Patient Bearing Devices
· 17 Dental instruments
· 22 Clinical Examination Instruments
In the list these items are explained:
· Product code
· Product description
· Intended use
· Example
· Product classification
· Pathway:
1. CER based on exemption catalog
or
2. CER based on equivalent or similar devices
or
3. or 4. Clinical study overseas or in China
As example of 13-xx-xx Passive Implantable Devices, in the list 18 product codes are chosen for clinical study (Pathway 3 or 4), 20 product codes based on clinical evaluation with equivalent device (Pathway 2). and 14 product codes are exempted from clinical evaluation (Pathway 1). All of 52 listed product codes belong to class III medical device.
In our blog we list all product codes of medical device needed for clinical study under this group.