China competes against US in medtech (NMPA & FDA)

Background
This article is written for all personal interested and working on 2 major medical device markets in the world China and US. Unlike politic conflict, there are many friendly similarities in these 2 most complex agencies from legislative to registration.
We want to compare US and Chinese markets of medical device in an easy and competitive way. From our view, Chinese authority National Medical Products Administration (NMPA) has followed a lot from the most modern U.S. Food and Drug Administration (FDA) and expanded and improved even a bit with Chinese elements.
Actually NMPA had a former American name Chinese Food and Drug Administration back in 2014 when the basic legislative is in form initialled. Since 10 years the regulations are developing in a crazy rapid tempo and high-quality regulating tone.
Compared to NMPA, FDA has its Federal Food, Drug, and Cosmetic Act (FD&C Act) already in 1938. Its legislative and standards are still state of the regulatory art in global compliance.
Registration scores (USA & China)
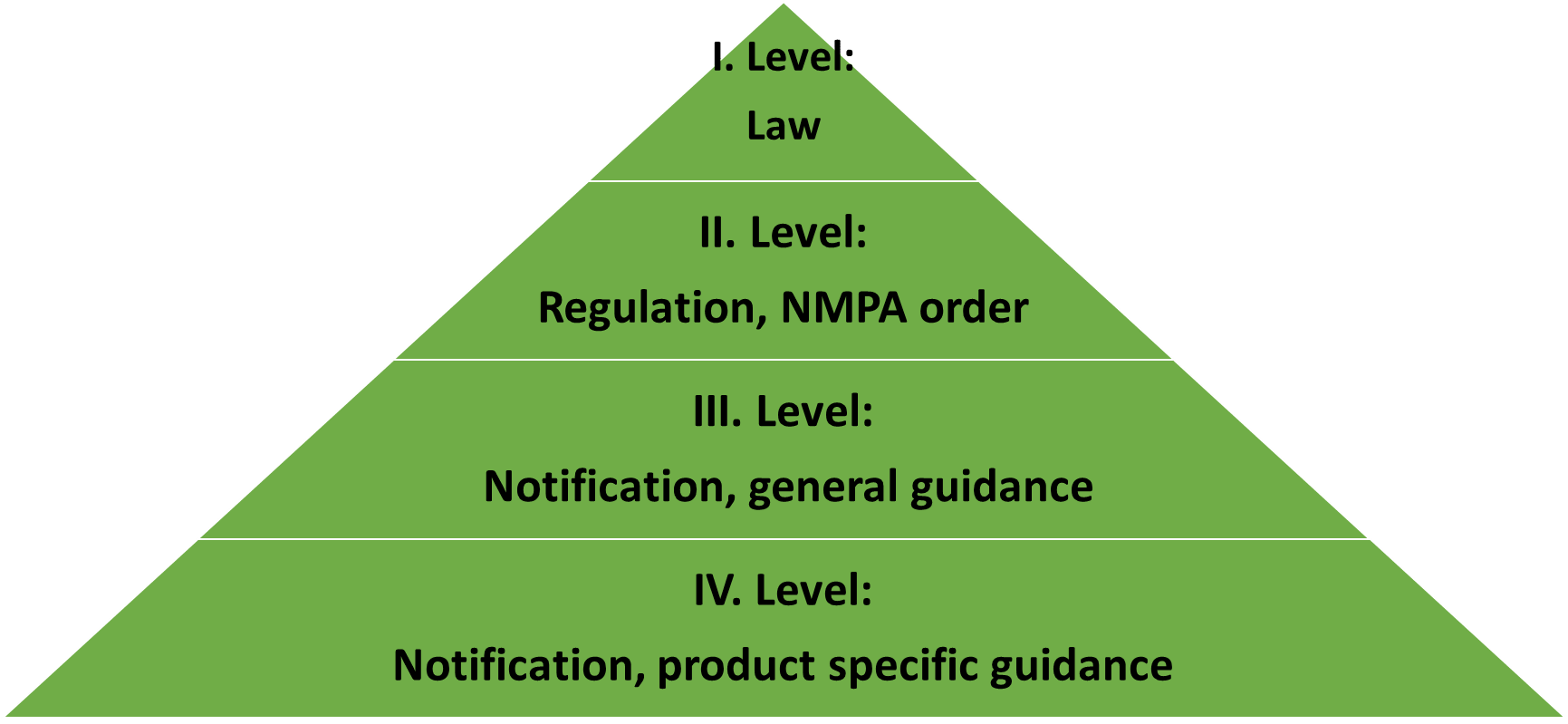
1. Legislative, 1:2
In US the law of medical device is under Title 21, Chapter 9 and Subchapter V: Drugs and Devices in FD&C Act by U.S. Congress. Code of Federal Regulations (CFR) is published by assigned federal regulatory agency (FDA). The reference of medical device is in Title 21, Parts 1-99 and 800-1299. There are 2 resources to look after law of medical device which is mixed with drug often in US.
The only binding law of medical device in China is newest regulations on Supervisory Management of Medical Devices in 2021. Since then diverse provisions, notifications and guidances are published which should be comply with and followed in the whole life cycle of medical device marked in China.
There are supporting guidance and standards stipulating the requirements of medical device in both markets and play critical role around product development and registration.
Due to Chinese language and regulation sometime brief formulated and in lower edition it makes difficult to understand Chinese legislative. In term of difficulties of legislative China wins.
2. Quality management system, 2:1
In US the quality system is written under 21 CFR 820 which is transforming to ISO 13485 now.
In China for products manufactured domestic, manufacturer has to apply for Chinese GMP compliant to “Measures for the Supervision and Administration of Medical Device Production”. For products manufactured abroad an overseas evidence of quality management system is needed for product registration only, as ISO 13485 or QSR in FDA.
From the view of foreign manufacturers, entering US market means to align additionally to 21 CFR 820 with extra efforts. US beats China with more QM effort.
3. CN & US agent, 1:1
For foreign manufacturers in both markets, you need a legal person there. In US it is called US agent, in China of course Chinese agent. They are quasi a regulatory representative for foreign manufacturer. From our practise, both agents are essential to submit electronic technical documentation and to communicate with authority by dossier deficiency.
In the reality after product approval, Chinese agent has more actions to coordinate as vigilance, to register of UDI and issuance code. Normally foreign manufacturer completes after approval actions on its own in US, maximal with help of US correspond.
4. Product code and classification, 1:1
In US there are 16 classification panels with 7-digits regulation numbers covering 1700 product types. Parallel there is 3-digits product code.
China has transformed product code from US to a 6-digits product code. Behind 6 digits of product code it hides three category levels. There are altogether 22 main product groups.
Knowing regulation number and product code in US you can find classification, submission type, standard, GMP requirements.
Knowing product code in China you can match classification, guidance, Chinese standards and clinical pathway.
On both markets there are risk based 3 classes I, II and III which is determined by intended use of medical device.
The higher the class the product has, the more complex is technical documentation to submit. For
class I medical device in both markets there are only
filling system
without review process.

5. Technical document, 1: 1
Now we are at stage to submit electronic technical documentation. Every market has own electronic system and folder structure to file technical documentation. We want to limit the comparison in typical class II/ III medical device initial in China with US 510k (premarket notification).
Surprisingly under main sections or chapters between US and Chinese market there are huge similarities.
For US 510k it is declared as substantial equivalence to another legally U.S. marketed device. This equivalent philosophy is at chapter comparison with predicate device and clinical evaluation in China.
The requirements for technical documentation in both markets are transparent and predicable.

To master product registration, it is the key to know the heavy weights to be examined by authority. We explain below product testing and clinical evaluation.
6. Testing for registration, 1:2
The regional testing is time -and cost consuming. In US you have to send device to US undergoing usability validation with US-citizen. For radio wireless products you should not forget the testing with Federal Communications Commission (FCC).
In China for class II and III medical device, manufacturer has to send device to China by from state accredited testing institutes. No testing unit should fail with operation of Chinese engineers at testing institutes. The performance and functional specifications should not deviate than required in national and industrial standards or by manufacturer own stated.
It is challenging to ship medical device for foreign manufacturer to China to be tested compliant with Chinese standards which is not always aligning with newest international standards. Regarding product testing for registration, China beats US with more challenges.
7. Clinical evaluation, 0:1
In US there is no clinical evaluation. For some high risk devices, clinical study is needed.
In China the requirements about clinical evaluation is similar but lower than Europeans clinical evaluation (MDCG 2020-13). For clinical evaluation manufacturer has to compare with approved own or third party equivalent device in China. As well only for some high risk devices, manufacturer has to perform a clinical study in China. Often overseas clinical data is recognised in China.
Since in China you should edit a special clinical evaluation with Chinese requirements, the clinical need is more demanding in China than in US.
8. Review and reply time of TD, 1: 1
We compared the review time by authority of typical registration at FDA 510k with class II and III medical device in China.
As seen in figure within 100 working days for 510k and within working 180 days of PMA, FDA should complete its review. In China for class III the review time is almost the same as for PMA in US. Chinese authority needs longer (155 working days) for class II medical device than 510k.
Regarding reply time of deficiency on site of manufacturer, Chinese authority allows less strict one year time to reply in contrary to 180 working days for additional information in US.


9. Official Cost, 1:1
We compared the official cost for typical registrations in China and in US.
In US for small business the cost is economical. Besides for Establishment Registration, manufacture has to pay annually, in 2024 $ 7,653.
In China we list price of typical registrations below. Change registration is as new 510k upon substantial change. Because the Chinese certificate is only 5 years valid, it must be renewed every 5 years.
| Product Class | Type | Official cost (Dollar) |
|---|---|---|
| Class I | Initial and change registration | Free |
| Class II | Initial registration | 29.700 |
| Change registration | 5.900 | |
| Change registration | 5.900 | |
| Extension registration (every 5 years) | 5.750 | |
| Class III | Initial registration | 43.500 |
| Change registration | 7.100 | |
| Extension registration (every 5 years) | 5.750 | |
| Clinical trial | 6.090 |
10. PMS, 1:0
In term of overseas inspection so far there are more possibilities to be inspected by US authority than Chinese authority on site of manufacturers. However after medical device is placed in China, in all supply chains products can be chosen to test upon requirements already proved at registration.
In term of vigilance there is different deadline and process in both markets unfair to distinguish.
In term post market surveillance, in US is more passive in product risk-based approach. FDA can order two primary types of mandatory postmarket studies: so-called “522 studies” (also referred to as postmarket surveillance studies) and PostApproval Studies (PAS).
In China manufacturer has to actively submit annual quality system self-report and Chinese PSUR which is part of after approval activities.
Summarised after product approval, manufacturer has to maintain post market more intensively for potential FDA than NMPA. In China there are increasing post market monitoring. It is still not that difficult to get brief report approved.
Conclusion
The final score is US & China 10: 11. We highly recommend manufacturer to welcome these attractive markets. Through a demanding registration is best way not only for future sales making innovative products quicker to patient but also a chance to prove safety and effectiveness to generate next product generation.
Do you want to know more insight more than these main registration factors, you can visit our one year academy with personal coach.
How about to have us as interim manager? Understanding global landscape of compliance is our enthusiasm. We wish to break next registration record with you.