Registration steps of medical device (IVD) in China
Mycompliance eLearning of regulatory affaris with Video, Test, Coach.
eLearning can be visited any time, valid for 1 year.
Participation possible any time, 24 hours a day, 7 days a week
Authority of medical device registration in China
Chinese medical device evaluation (CMDE) conducts technical review of medical device in China. The new legislative regarding medical device is published at website of CMDE, although its superordinate authority National Medical Products Administration (NMPA) is best known and mentioned.
Unfortunately, the website is not translated in English. However, CMDE has an elegant work flow with most important steps of medical device registration in China in English. It is perfect for beginner even for experienced RA specialist to study interested topics behind the work flow.
If you wish to download original Chinese regulations in interested “registration subjects”, you have to go back to Chinese work flow with help of some translation tool.
Workflow as an overview
Workflow in video explained
Workflow in detailed scripts
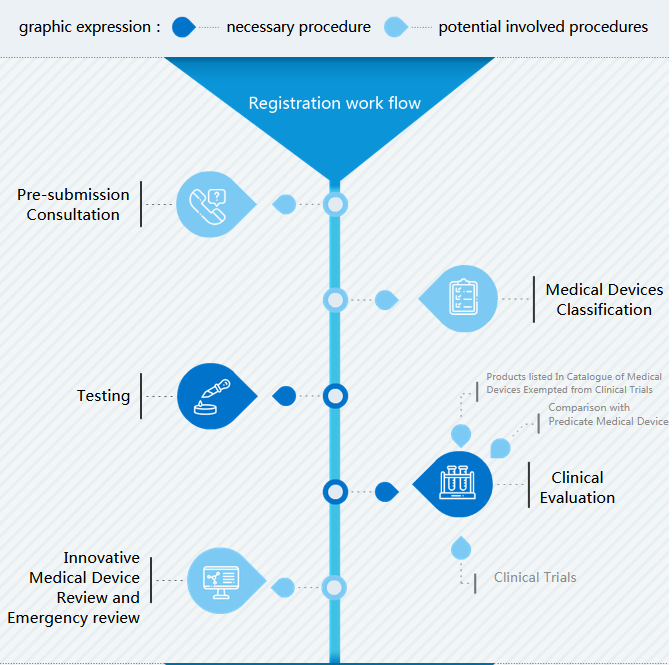
Workflow of medical device registration in China is made of three parts:
- Registration work flow with important steps
- Review and approval at site of NMPA (premarket)
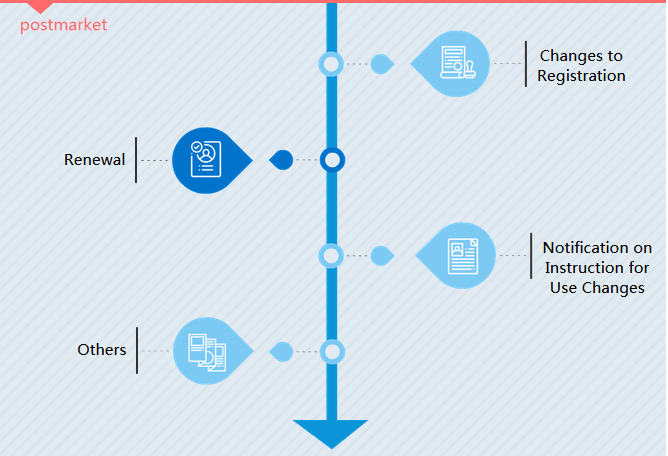
- Registration type (postmarket)
- I Registration: The important step in prior to preparation of submission dossiers is to determine classification. During the preparation of medical device registration in China are type testing and clinical evaluation the most challenging parts.
- II Review and approval: After submission of technical dossiers, CMDE undertakes acceptance review at which completeness of documents is proved and technical review after which supplementary info is asked.
- III Registration type: After initial registration it is manufacturer`s turn in 5 years to renew the product certificate. If medical device has some significant changes, a change registration is needed.
I Registration
Medical Devices Classification
There are I, II and II medical device in China. The best way to find classification is to match product code at classification catalogue.
If the classification is not able to be determined, manufacturer can directly register medical device as class III or apply for classification determination at NMPA. If it was a “special” medical device, an innovative pathway can be used.
(Type) Testing
Object of type testing is class II and III medical device. Manufacturer has to draft product technical requirement and to select a testing center.
After the medical device is sent to China, the testing will be conducted.
Clinical Evaluation
Three types of clinical evaluation are possible in China: simplified CER if medical device is in catalogue exempted from clinical trial, CER comparing to similar products which are approved in China and clinical trials in China.
Registration Application Materials (=technical documentation)
The submission structure here is in new table of content format. The documents in this format should be submitted via electronic regulated product submission (eRPS).
At work flow are just shown main contents and documents in top 6 CH-folders.
II Review and approval
Acceptance of Registration Application
The acceptance review of submission dossiers is new because of some begiining chaos of electronic submission of dossiers via eRPS since 2019.
In subfolders (at level CH X.X.....), manufacturer should submit corresponding document, sometimes even a statement in documents if the content doesn’t apply to subject registration.
Every legal manufacturer who has a physical office and business licence in China, can apply for a certificate authority which accesses the use of eRPS system. For foreign manufacturer without its own Chinese entity, normally chinese agent takes over this role.
If the submitted documents via eRPS are complete, CMDE issues "Notice of Acceptance" and "Bill of Payment". Otherwise "Deficiency Letter" or "Notice of Rejection" are issued. By "Deficiency Letter", manufacturer has to re-submit the whole dossiers again.
Supplementary (same technical review, similar to US substantive review)
After technical review, manufacturer has one year time to deliver supplementary information according to the "Deficiency Letter".
The time of preparation for deficiencies will not be included in review timeframe. Manufacturer can consult once relevant reviewers.
III Registration type
Changes to Registration
There are two types of change registration. In CMDE language, they are changes of permission items (administration matter) and changes of registry items (significant modification matter).
In China there is no “letter to file” for insignificant change of product. However, a change assessment is recommended for internal process and in case of inspection by Chinese inspection.
The time of technical review at change of registry items (significant matter) by authority is the same (60 working days). For changes of permission items (administrative matter) the technical review takes only 20 working days.
Change registration medical device has the same certification number as at initial registration. Manufacturer should subsequently modify IFUs and labels by adding new issued certification number and updating manufacturing date of approved medical device (after certification issuing date).
Renewal
A medical device registration certificate is valid for 5 years in China. Manufacturers shall apply for renewal no later than 6 months prior to the certificate expiry date.
At time of renewal, extensive efforts as at change registration are required, if e.g. Chinese mandatory standards are revised. It means often new requirements to prove safety and effectiveness of medical device in term of new type testing which triggers firstly a new change registration in prior to renewal.
Video of registration process (official)
In this short video, CMDE gives you an overview of pre-submission, acceptance and technical review and consulation after deficiency letter in China.
Video of road map of Chinese registration
In this short video, we try to summarise the insights to begin a Chinese registration of medical device and IVD. For each topic around compliance look after the other articles or other elearning video.