Chinese Agent
= Marketing authorised holder (MAH), legal representative
A key Factor for Success of Chinese Commercialization of Medical Device
Chinese Agent for foreign legal manufacturers (imported medical device or IVD)
Chinese Agent is also called
authorized - or in country representative. It is equivalent to
US agent or
UK responsible person. The Chinese agent with business license in China is required for
every
overseas manufacturer
who has no entity in China. One manufacturer can assign as many Chinese agents as needed. However, only one Chinese agent per product is allowed. Chinese agent will be written on
NMPA certificate besides the name of legal manufacturer.
Please don´t be too overwhelmed after successful product approval. It is hundred percent your right to request all final
approval certificates (also "product technical requirement" as annex and type testing report) issued by authority. It facilitates the next
change - and extension registration as essential documents.
Tasks of Chinese agent
The regulatory responsibility and requirements of Chinese agent is increasing
Responsibility of Chinese agent:
• Compilation of the submission dossiers
• Communication with NMPA
• Report of adverse event
• Assistance of the potential inspection by authority
•
Transfer of regulatory update
New
· Upload of UDI-DI and insurance code in Chinese data base
Nice to have
· Assessment to significant change of approved medical device in China
· Prove of IFU and label
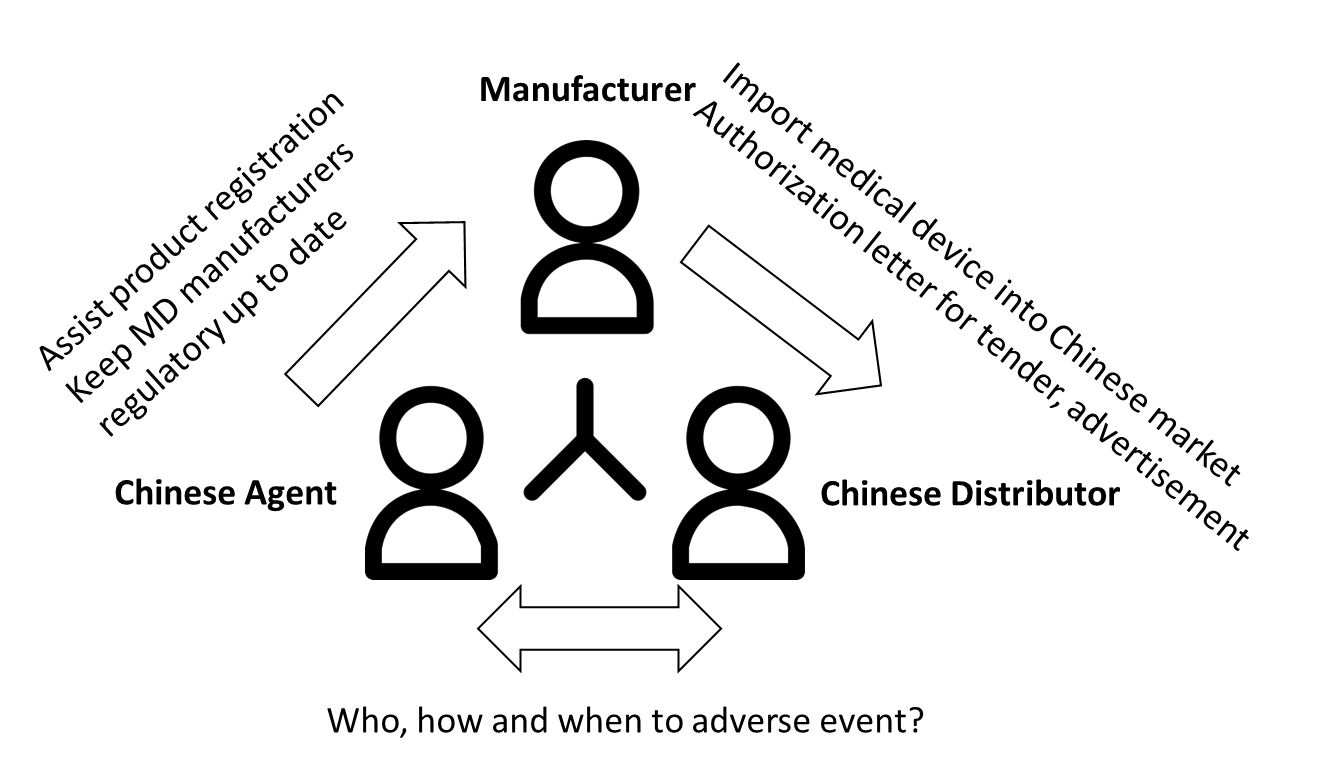
Controversial responsibility of adverse event reporting (tender) for overseas manufacturers
It is a dilemma who should actively coordinate adverse event at post market activities. Chinese agent focuses on registration and Distributor focuses on sales. At new issued regulation, NMPA strengthed the role of Chinese agent for adverse event reporting and maintenance of product distribution indirectly (as EU authorized representative).
The manufacturers are aware of reporting adverse event and recalls since 2019. It is easier for domestic manufacturers when the corresponding departments are among the same house. For overseas manufacturers it is far behind required how to coordinate adverse event - and recalls reporting. Chinese distributor should have agreement with Chinese agent how to handle reporting and to rule the accountability. It means that a procedure of receiving -, analysing -, reportig - as well as handling AE and recalls is needed.
We highly recommend to mandate this role with caution to distributor. On the one hand they don’t have the sound regulatory knowledge. In the most cases they find another consulting firm to do product registration. It exists Ping-Pong communication among three parties. On the other hand they oft keep hold of original
NMPA certificate to block the next product registration and even the process of changing Chinese agent.
Binding obligations of Chinese agent
At Regulations on the Supervision and Administration of Medical Device (State council order 2021/739) it stipulates:
Article 20
Registrants and record holders of medical device shall perform the following duties:
(1) Establish and adapt product quality management system and applies it effectively;
(2) Development of post-marketing studies and risk management plan and to ensure the effective implementation;
(3) Carry out adverse event monitoring and re-evaluation in accordance with the law;
(4) Establish and implement product traceability and recall system;
(5) Other obligations stipulated by the drug regulatory department of the State Council. The domestic legal person designated by overseas manufacturers shall assist the registrant and filing party in fulfilling the obligations stipulated in the preceding paragraph.
domestic legal person = chinese agent
Case study of Chinese agent
We have experienced many scenarios whom to assign to be Chinese agent. Since electronic certificate of medical device, the classic story that old agent block paper certificate to new agent doesn’t exist.
Scenario 1 Consulting as Chinese agent
Advantage:
Consulting has solid regulatory knowledge
Easy Switch to new distributor
Disadvantage:
Annual holding fee to pay for consulting
Complicated for adverse event communication and reporting
Scenario 2 Distributor as Chinese agent
Advantage:
Simplified adverse event management
Easy for issues related to medical device
Disadvantage:
Less regulatory experience
Moderate difficulty to switch either new agent or new distributor
Scenario 3 Combination of above, first years consulting, then distributor as Chinese agent
In the transition period you can test consulting and distributor who fulfills his responsibility well and then transfer slowly to reliable distributor
Our suggestion:
Scenario 3, if you chose us as regulatory partner, we can support either inexperienced subsidiary or external distributor
What to do if to change Chinese agent
A brief administrative change registration is needed in case foreign manufacturer wants to change Chinese agent. The following actions should be considered:
Update label and instruction for use in Chinese
Complete all adverse events with old agent and transfer the account to new agent
Update registering of insurance code and UDI
Ask all electronic product dossiers, info of adverse event, insurance code and UDI from old Chinese agent
Plan of next quality management annual report and periodic risk assessment report
Our Service
- Service of qualifing new agent or distributor
- Build of regulatory role at your Chinese partner
- Inspection of Chinese partner compliant to good manufacturing or distribution practice
- Provide checklist and questionnaire for your own selection of Chinese partner
- Interim manager to act any role at your Chinese entity
- Transfer overseas quality policy and product expertise in terms of training or workshop
- Project management
Related link of agent, authorised represenative, In-Country Representation, responsible person and marketing authorization holder (MAH) or registration holder
U.S. Agents
EU Authorised Representatives
UK Responsible Person
Contact us, we are expert of global registration too.