Type Testing of Medical Device in China
TYPICAL CHINESE and CHALLENGING Type Testing
Type testing is unique Chinese, it is also called in-country testing or product/sample testing. Similar testings can be found also in Russia, Japan and Brasil (Inmetro).
Besides preparing submission dossiers in paper at product registration, the foreign medical device must be sent to accredited testing institutes by NMPA in China, after drafting Product Technical Requirement (PTR) as seen in figure 1. Type testing applies to some of class I - and all class II and III medical device. The final testing report is one of the important documents among submission dossiers. The revised PTR with functional specifications will be reviewed by authority and issued as annex together with NMPA certificate after product approval.
The biocompatibility report, pre-clinical study, shelf life validation report, packaging validation report are beyond type testing and these reports are oft accepted by NMPA.
The workflow of type testing shows a really long process which might weight one third of time and effort in the whole product registration. The input of type testing is drafted PTR by legal manufacturers with potential chinese agent. After confimation by testing institute, medical device can be sent to China. Normally it is better to chose one skillful engineer and a Chinese spoken expert to accompany the test. They can operate medical device and answer questions directly at testing. Parallel by waiting for testing report, the manufacturer can start the preparation of main submission dossier.
At technical review by NMPA the testing report and revised PTR will be proved as well.
If you complete successfully type testing, it seems to finish the toughst part at "marathon" of product registration in China!
New
As of 2021 the type testing and testig report by qualified own - or third party testing institute are accepted by NMPA
Procedure, Time, Cost and Documents for type testing
1. Procedure
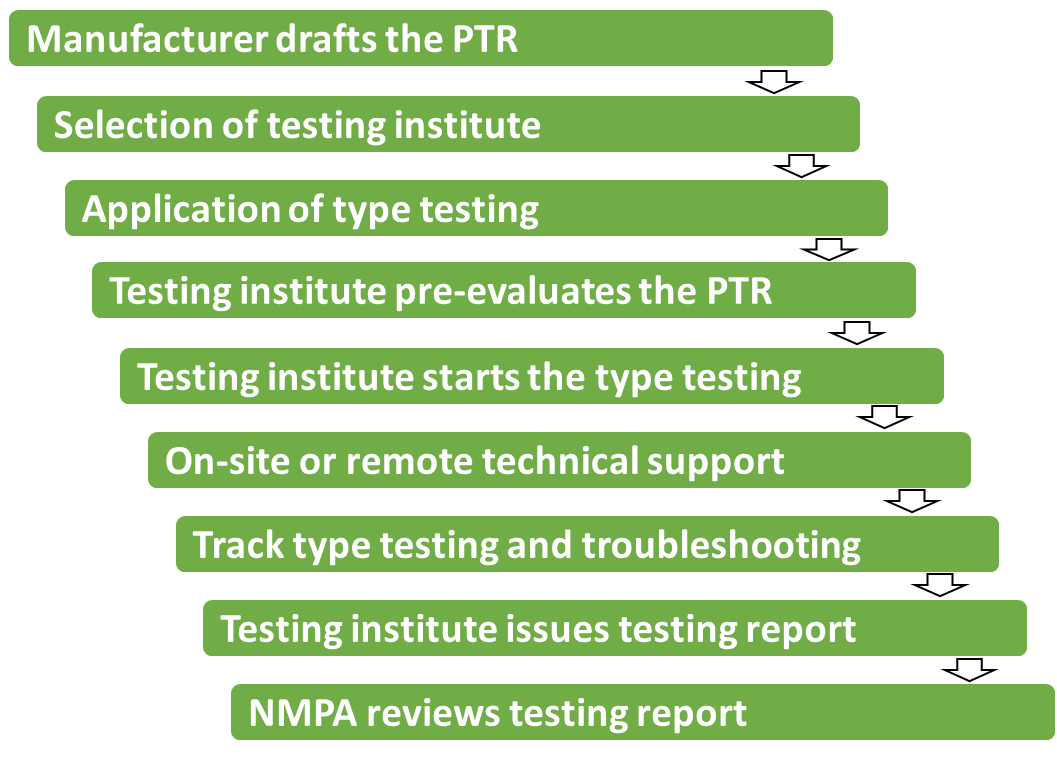
In figure 2 the important steps are illustrated at type testing. The contact person is mainly testing engineers at testing institute. Even during the technical review by NMPA the reviewer would have an exchange with testing engineers regarding the testing details. After the application of type testing, the drafted PTR will be evaluated by testing institutes before legal manufacturers sign an agreement with them.
Overseas manufacturer can then prepare the sample for testing, import it in China and even pre-test the sample simulating difficult testing scenario in home country. Normally because of high demand of testing there is long waiting time till exact date of type tetsting.
2.Time
Waiting time in queue before type testing
After application of type testing: 3-6 months depending on testing institutes.
Not all medical devices are in the scope of the capability of testing institutes. In this case the manufacturer should apply at central NMPA for authorizing this test to testing institute in which the application takes around 1 month.
Time of conducting type testing: 3-6 months depending on product type
* The time could be longer in case. There is unpredicted trouble shooting at site of manufacturers. There are always queue of diverse testing unit which must be followed each other instead of testing parallel.
On average, the testing of stand alone software and passive MD are less time-consuming than electrical medical equipment. For last one, the safety - and electromagnetic compatibility on-site test have to be conducted in different testing units.
3. Cost
The cost of type testing varies in 5k-20k EUR depending on product type and testing institutes.
4. Documents
- PTR
- Instruction for use in Draft (Chinese)
- Draft Labels (Chinese)
- Application form
- Risk management
- Usability test (if appicable)
Active electrical medical device:
- IEC 60601-1 and IEC 60601-1-2 report in ENG
- Certificates of critical components
- EMC contents (product components, power supply cables, list of critical components, supporting accessories for testing)
- Electrical schematic
- Circuit diagrams
Some problems, thoughts and even illusions about type testing
It is not exactly comprehensible which key performance specifications are essential for type testing if there was no product-specific guidance or standard. It is also obviously unequal which core scope different testing institutes are specialized on. It results in some “unreasonable” trouble shooting during the type testing. Find the reliable consulting partners with experience of interested product group and better with strong network with testing institutes.
At the moment, NMPA is aware of unavoidable disadvantage of type testing. They try to ease the policy to allow more qualified third-party testing institutes stepping in and in new notification accept self-report of testing. Hopefully one day the type testing will be shortened in range or abrogate by accepting most or all overseas testing report by accredited testing laboratories. It is speculated that the testing report of medcial device by Chinese manufactuers will be accepted at globe authority at the same too.
PTR and type testing are typical “made in China”. For overseas manufacturers this testing is an absolute deceleration during the product registration in China. Without doubt it is mainly a repeated testing or even eximination covered by internal verification tests and other tests by extern testing laboratories as view of overseas manufacturers. However, it exists indeed some differences of Chinese and international standards which demands additionally.
Be aware that in the Chinese life cycle at supply chain, authority has each year focused products to be inspected and applicable testing items in Chinese standards.
Do you want to fully understand local testing in China, context bw PTR, risk management and testing?
Visit our E-Learning and on demand workshop which reveals not only the insight of type testing, you have also learning video, script, free consulting minutes, case study and free template.
Are you good at chinese testing? Make a self-test (part of e-E-Learning), we show some tips and results