Electronic Adverse Event Form
Backgroud of adverse event reporting in China
Since 2019 NMPA strengthed the monitoring of adverse event reporting in China. The supporting regulation are Measures for the Administration of Monitoring and Re-evaluation of Medical Device Adverse Events and Guidelines for medical device registrants to carry out adverse event monitoring.
We highlight the original Chinese "Marketing Authorization Holder Medical Device Adverse Event Report Form" below and provide the only replying parts of analysing adverse event in English for foreign manufacturers which should be completed by Chinese agent (same as Marketing Authorization Holde in original form in Chinese and be submitted in database of adverse event in China. These post market activities are) passive or reactive reporting after the health institutions or distributors have registered the adverse event in database of authority.
Excepted cases below discussed are
· Group medical device adverse events
· Active reporting of adverse event outside China which should be collected by foreign manufacturers
Chinese report form of adverse event at database
注册人医疗器械不良事件报告表
Marketing Authorization Holder Medical Device Adverse Event Report Form
报告基本情况
报告编码:
报告日期:
报告人:
单位名称:
联系地址:
联系人:
联系电话:
发生地:
医疗器械情况
产品名称*:
注册证编号*:
曾用注册证编号:
曾用注册证编号上报:
型号:
规格:
产地*:
管理类别*:
产品类别*:
产品批号:
产品编号:
UDI:
生产日期:
有效期至:
不良事件情况
事件发生日期*:
事件发现或者获知日期*:
伤害*:
伤害表现:
伤害表现附件:
器械故障表现:
器械故障表现附件:
姓名:
出生日期:
年龄类型:
年龄:
性别:
病历号:
既往病史:
使用情况
预期治疗疾病或者作用:
器械使用日期*:
使用场所*:
场所名称:
使用过程*:
合并用药/械情况说明:
事件调查
是否开展了调查*:
调查情况:
调查情况附件:
评价结果
关联性评价*:
事件原因分析:
事件原因分析附件:
是否需要开展产品风险评价*:
计划提交时间:
控制措施
是否已采取控制措施*:
具体控制措施:
具体控制措施附件:
未采取控制措施原因:
错报误报
是否错报误报*:
错报误报原因:
错报误报原因附件:
报告合并
是否合并报告*:
合并报告编码:
报告审核情况(上报地设区的市级中心)
审核结果*:
审核意见:
审核人:
审核单位:
审核日期:
评价审核情况(注册人所在地省级监测机构)
审核结果*:
审核意见:
审核人:
审核单位:
审核日期:
事发地省级意见
意见:
填写人:
填写时间:
评价复核情况(国家监测机构)
复核结果*:
复核意见:
Analysis part at adverse event form to submit
The key effort for foreign manufacturers is to have a analysis of adverse event with help of Chinese distributors and health institutions. The control measures could even not be started at stated below.
Were site surveys carried out?
Survey status:
Relevance assessment:
Cause of incident analysis:
Whether a product risk evaluation was required:
Planned submission date:
Control measures in place or not:
Specific control measures:
Reason for not taking control measures:
Whether misstatement or misrepresentation.
Reason for misstatement and misreporting:
Whether reporting is consolidated:
Consolidated reporting code:
Keywords to report Chinese adverse event
- Adverse event report form in China is only in Chinese.
- All stakeholders in supply chain of medical device could submit an adverse event.
- All submitted adverse events are automatically serious adverse event.
- The serious adverse events are part of Chinese PSUR.
- Legal manufacturers has right to recall misstatement or misrepresentation.
- Only Chinese manufacturers or Chinese agent for foreign manufacturer could complete the form and submit it to authority.
- Normally there is a review of adverse event firstly in a city -, then provincial level of monitoring agency, followed by central NMPA at the end.
- The form is made of chapter filled by applicants, manufacturers and reviewer at authority.
Workflow of Chinese adverse event
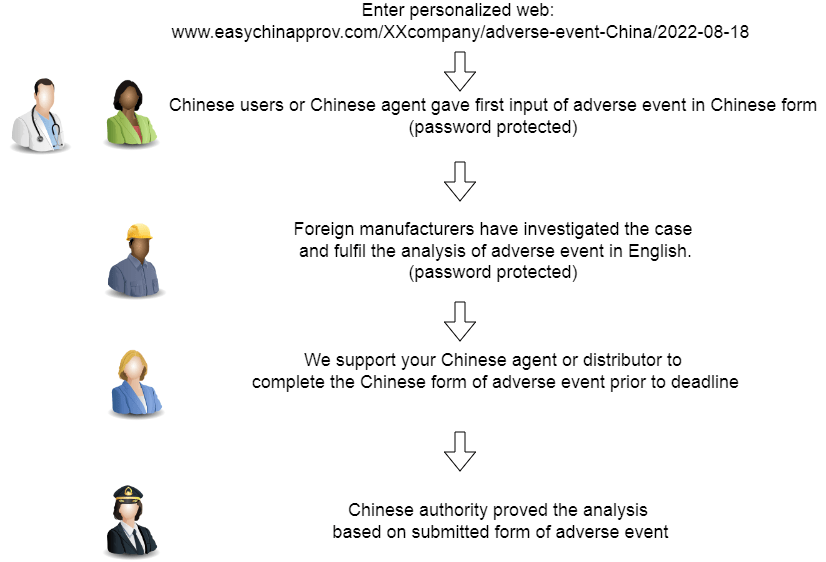
Easy adverse event form
We are making adverse event report faster.
With similar principle we have the automated form to report group adverse event or recalls to Chinese authority. We are keen to support complaint reporting from users to reduce workforce of your vigilance department too.
Our advantage of unique tool:
· You can use our form as your outsourced vigilance database on your name.
· Manufacturers and Chinese stakeholder has each EN and CH instruction to fill the form without vigilance knowledge.
· All Chinese stakeholders could fill or upload the form in Chinese which will be translated in English.
· During the analysis of adverse event, a reminder is sent to guarantee to submit the final report at Chinese authority prior to deadline.
· At the end manufacturers would have a complete report of adverse event in English.
· Among requests, we could communicate to your Chinese partner and with you.
· We could integrate your vigilance process and content in our electronic form so you have a solid database to generate global history of adverse event.
Use your first adverse event free with our tool. After the convincing results you decide how to proceed.





