Trace Chinese Registration
Traceability Matrix at Chinese Registration
Keywords
Digital tool, registration monitoring, submission assistent, submission compilation, digital project management, design transfer, AI translation, design history file, technical documentation, regulatory lean, regulatory science, matrix in reglatory affairs, feasibility of global registration, requirement traceability, risk management, clinical evaluation, NMPA, MDR, MDSAP, FDA
Why do you need traceability matrix for Chinese registration of medical device?
The easiest matrix at Chinese registration might be communication matrix in project, order and invoice.
We want to focus on some premium matrixs which are for manufacturer time - and budget consuming for success and play definitely a great role for compliant registration and follow up tracing next generation`s development of medical device (IVD).
We analysis the traceability matrix dedicated for foreign manufacturer who has a bit mind gap due to regulation and language difference to Chinese market.
We use the same mindset changing for another global regstration and international project using digila tool.
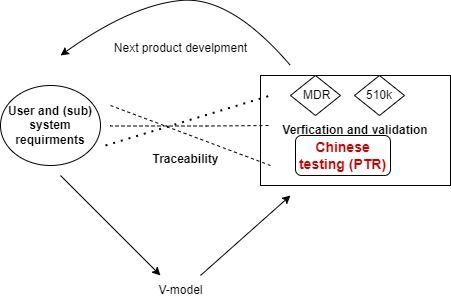
1. At whole development lifecycle
Key specifications are traced with user requirement, risk management, V&V, product description, Chinese IFU and Chinese testing
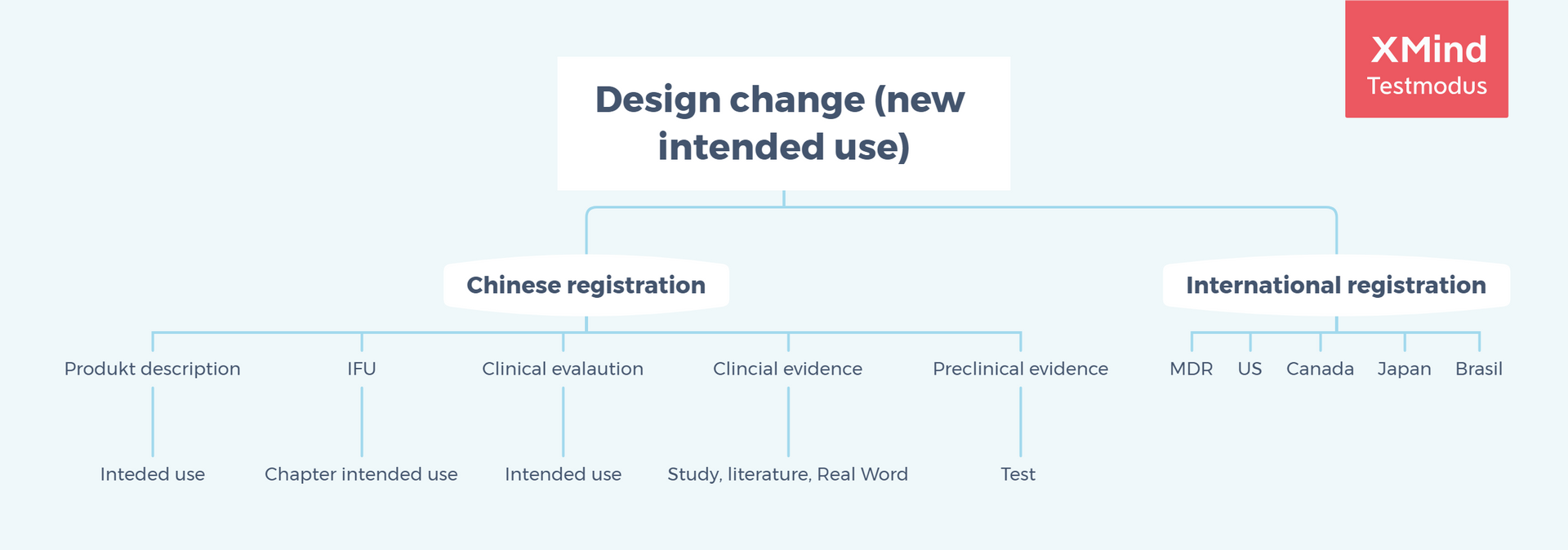
Design Change should be related to impact on new change registration, exact Chinese technical documentation, change comparison table, Chinese IFU, and even annual quality management report for overseas manufacturers
2. At registration dossier
Intended use should be consistent in Chinese certificate, Chinese IFU, device description, supporting evidence, and clinical evaluation.
Key risk requirement should be consistent in specification (and/or symbol and warning text) in Chinese IFU, label, verification and validation.
Especially at change registration, describtion should be consistent, risk management, labelling and evidence report should traced.
3. At review stage by Chinese authority
Deficiency question should be traced with exact chapter at technical documentation as reply, potential update in Chinese labelling and design history file (perhaps new feature in next product generation).
Ignored at authority is that deficiency at product regitration is best finding practise to correct mistake or improvement in running or palnned product realisation.
4. At post market after Chinese approval
One complaint or adverse event case can trigger risk management, CAPA, reporting in Chinese vigilance, part of annual quality management, periodic risk evaluation report and overseas inspection
One important requirement matrix: product technical document for Chinese type testing
Manufacturer of all classes medical device has to send device to test in a certified lab in China. As an important document for type testing is product technical documents (PTR). It is very similar to input for safety, EMC or IEC 62304 etc. testing, however it will be drafted FIRSTLY by manufacturers with extreme summarised contents (less than 10 pages usually). The special features in PTR are:
• PTR is an input and output for Chinese type testing
• Manufacturers could actively define the specifications to test
• Obligatory Chinese standards require what and how to test
• Applied standard as method could be Chinese, international recognised or suggested by manufacturer
• Finalised PTR is part of technical documentation to submit to authority and acts as annex for Chinese certificate
• PTR is an important resource for design change after approval
There are 2 important chapters in PTR: chapter 2 performance specifications and chapter 3 method.
Each specification in chapter 2 is to match to corresonding method in chapter 3. This is ignored by overseas manufacturers especially. These requirements should be controlled in the whole lifecycle of medical device, not just for registration.
Here is an example of PTR showing only 2 specifications: (downlod the whole PTR and see other templates)
2.1 Working conditions
2.1.1 Environmental conditions
The working environment conditions of the product should be clarified, such as: ambient temperature, relative humidity, atmospheric pressure, etc.
2.1.2 Power supply conditions
The power supply voltage, frequency, resistance and power capacity of the product should be specified.
Shall comply with YY / T 0706-2017 of 5.2.1 requires clause.
2.2 Electric power
2.2.1 Maximum output power
The range of maximum output electric power should be specified.
Shall comply with YY / T 0706-2017 of 5.2.1 requires clause.
2.2.2 Nominal output power
The range of nominal output electric power should be specified.
Shall comply with YY / T 0706-2017 of 5.2.2 requires clause.
3. Testing method
3.1 Working conditions
Should meet the requirements of 2.1.
3.2 Electric power
3.2.1 Maximum output power
Press YY / T 0706-2017 the 6.2.1 test method provisions.
3.2.2 Nominal electric power
Press YY / T 0706-2017 the 6.2.2 test method provisions.
The specifications at testing of Chinese registration don’t come from nowhere. It is part of design and development, namely in design history file. So it can be traced with:
• user requirements
• verification and validation
• risk management
• applied standards
• design change

What can we support to manage Chinese PTR requirement?
There should be an intelligent testing matrix at Chinese registration with design and development at manufacturer.
It is ideally to have a PTR matrix in an electronic tool.
Contact us in term of PTR demo in a smart tool to manage your testing matrix. We have established a unique solution with partner (Matrix Requirements). We can adapt your internal user need, guarantee the testing in Chinese and international registrations and control design change at quality management.
Here a simplified example of Chinese type testing matrix requirement:

Other Matrix should be traced for a successful project
Either in China or in EU or global markets, manufacturer should always keep in mind to have different types of traceabilities between requirements, specifications, technical documents, international dossiers, deficiency reply within diverse communication parties.
It matters because a good matrix in medical device field would:
- Make complex design and development transparent
- Accelerate variant development upon the same product group
- Leverage prioritised verification and validation in next product generation
- Trace critical standards or regulations with actions
- Evolve multi-department and multi-entity cooperation
Here is another matrix in aspect of design control (e.g. intended use) at quality management in post market.
Either for regulatory affairs (registration) in China or beyond, if you have a solid Excel or Word file with tracing items, have a free meeting with us. We will make a GRATIS matrix in customer project. You decide the next step and success.
Are you start-ups and do you want to start to establish a global regulatory affairs group?
You can also enroll our one year eLearning of regulatory affairs. We have special price.












