E-Learning of Regulatory Affairs (Chinese registration and beyond)
Welcome to
E-learning of Chinese and global Compliance of medical device
Unique feature of E-learning
- Free one to one supervisor with regular on demand meeting
- Test and homework with professional personal examination and feedback
- Possible expert opinion for current registration and other strategy
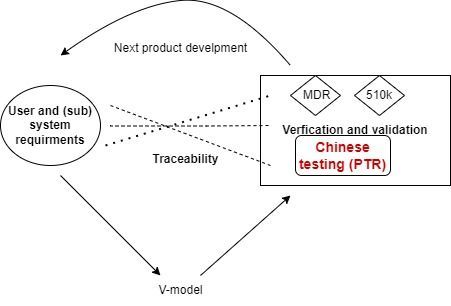
- Update of new Chinese registration, videos and templates
- Session 1: Chinese access
- Session 2: Registration in China
- Session 3: Chinese Post market and quality management
- Session 4: Global markets
- Session 5: Regulatory Affairs Soft Skills
E-Learning can be visited any time, valid for 1 year.
Participation possible any time, 24 hours a day, 7 days a week
Testimonials
Keyword
Medical Device, in vitro diagnostic, State Council Ordinance, China Food and Drug Administration National Medical Products Administration, NMPA, technical document, risk management, verification and validation, RPS, UDI, change registration, marketing authorization holder , overseas manufacturer, outsourced manufacturing , type testing, product technical requirement, clinical evaluation, instruction for use, labels, adverse event, vigilance, Quality management system, 医疗器械, 指导原则, 质量抽查, 说明书和标签, 体外诊断试剂, in vitro diagnostics, MDR, FDA, MDSAP, conformity assessment, ANVISA, Kaizen, global registration, regulatory intelligence, webinar, online university, template, on-demand workshop, roadmap, interim regulatory affairs, project management, product management, international product launch, ventre capital
Learning audience
- Student and unemployed:
Basic knowledge for Chinese registration
- Non Medtech personal:
Gain overview and tactic of Chinese market entry, useful for publication (medical writers)
- Non Regulatory personal:
Perfect for career changer
- Regulatory professionals:
Either with or without experience of Chinese registration, improve registration efficiency, ideal as boot camp for RA group
Leaning objective
- Regulatory Affairs (registration) of medical device and IVD in China and beyond
- Technical documentation in Chinese requirement
- Start from EU and US to Chinese dossier
- Quality management, quality assurance, project management, labelling, production, post market in term of Chinese registration
- Intelligent Leverage of international registration
- Soft skills of international regulatory affairs
Proof of E-learning
A certificate will be issued after successful test for each session and qualitative homework.
Example of one session in E-learning
In each session it is made of learning article, videos and test. Depending on your demand, you can ask for a supervisor minutes.
Open Videos to share knowledge
We try to share open sources of valuable video explaining the keys of Chinese and global regulation. The professionel version is coming up. Register as soon as possible because the early bird has cost benift and possibility to book trainer online.
Start: Participation possible any time.
Duration: 1 year
How to start: Kick off meeting with trainer, book training
End of E-Learning: for professional user there is test, free supervision hours and certificate
1. Insight of essential webistes with regulation of medical device and IVD
The following video share important Chinese websites where you can explore the different topics. Try to bookmark the links to monitor the useful contents using English translation.
2. In-country representative (Chinese agent)
Do you know that you need an in-country representative in China to register your medical device or IVD? It has also a smart name "Chinese agent". It is similar to "US agent" and plays a greater role than authorised representative in EU preparing, submitting dossier, replying deficiency and assissting post market to authority. It has little to do with importer and distributor.
Watch the video and be clear finally with your Chinese partner.
Payment
It is payed at the beginning of eLearning, valid for 1 year.
Cancellation is possible in 14 days free and with refund of 80% course payment (minus 20%) in 2 months. The cancellation is not valid if more than 2 sessions in professional compliance module was completed or template was sent to client already.
The enrolment of course could be transferred to second person upon our approval.
Early bird cost: 19 % discount
Standard MyCompliance
999 EUR
· + 40 topics
· + 20 Videos
· Multi choices Test
· 90 Min free consulting (one to one)
Professional Mycompliance
1499 EUR
· + 40 topics
· + 20 Videos (Update)
· + 20 case study Assessment
· Multi choices Test
· Update of new Chinese regulation
· Free template + 10
· 200 Min free consulting (one to one)