Chinese IFU and Label
Life cycle of Chinese IFU and Label

Requirements on Chinese Instructions for use and label by NMPA
In accordance to Article 39 at “Regulations on the Supervision and Administration of Medical Devices” (Decree of the State Council No. 739, 2021) and “Guidance of Instructions for use and labels of medical device” (order 6, 2014), basic requirements on Instructions for use and label have to to be fulfilled in China. Compared to article 23, chapter III, annex 1 at MDR in EU, the requirements on Chinese Instructions for use and label are a kind thinned out and quite basic.
However, some items on Chinese Instructions for use and labels are often ignored and keep a potential finding by medical device authority NMPA in China. The reasons are diversified:
Sometimes the sources of translations of Chinese IFU and labels are different: internal marketing - and RA department, external translation office, local consulting company or own subsidiary in China and distributors in China.
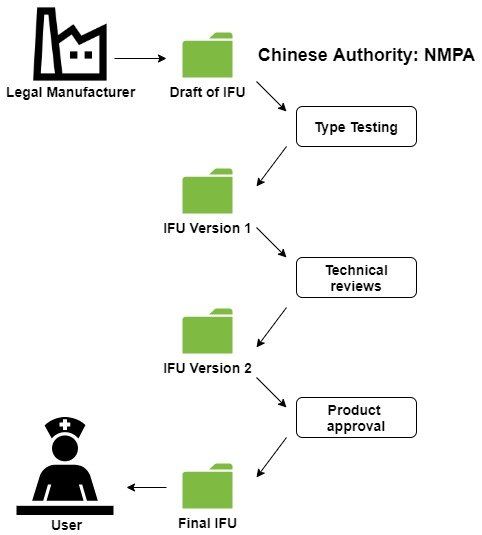
Unfortunately it exists no controlling system of Chinese IFU by legal manufacturers consistently. Besides it, the registration process from dossier preparation to product approval takes almost 1.5 to 2 years for e.g. class II medical device. Actually there should be at least three revisions of Chinese IFUs in time manner, namely after type testing, after technical reviews by authority NMPA and after final issue of NMPA certificate.
Another close related topic is symbol. In China manufacturer should have the exact wording compliant to standard YY/T 0466.1: 2007 Medical devices. Symbols to be used with medical device labels, labelling and information to be supplied. Part 1: General requirements (draft in 2022).
What happens if the final IFU is inconsistent with the content on NMPA certificate after product approval?
In case there are inconsistent contents at Chinese IFU, labels with submission dossiers or NMPA certificate. You might have problem with distributors placing medical device in Chinese market, the hospital could refuse the medical device by controlling goods receipt even after signing the sales contract. In the worst scenario, NMPA finds this deficiency out and gives a penalty to legal manufacturers.
Before finding by NMPA it is so far not complicated to make a rapid application in term of change registration of IFU which dosen`t cost at NMPA and it take just some weeks, if legal manufacturer revises IFU without changing significant contents as intended use, key technical parameters etc.
Life cycle of Chinese IFU
From plan of submission dossier to delivery of final IFU to client after product approval in China
We suggest that legal manufacturer uses only one resource of translation of Chinese IFU and labels with controlling system. As in workflow below (just IFU as an example), the life cycle of IFU starts from a draft of IFU. This is at the same time an input for product technical requirement (PTR) for type testing at which you have to submit IFU officially first time. Make sure that product name, variants/models and key technical parameters are at least consistent between IFU and PTR. Very often overseas manufacturers take care of draft of Chinese IFU. Chinese agent who is responsible for submission dossier, concentrates on PTR at basis of English IFU. The inconsistency happens not rarely.
After type testing the Chinese IFU might be revised if the technical parameters vary than given at PTR. Then it can occur that during technical review of submission dossier, some inconsistent contents, incorrect formulations and other performance claims are suggested by reviewers. In this case, the second revision of Chinese IFU is to follow.
At the end, NMPA will issue NMPA certificate with certification - and PTR number (the same) which should be added on final IFU. Meanwhile you should also leave revision date on final Chinese IFU.
Checklist of Chinese IFU and label
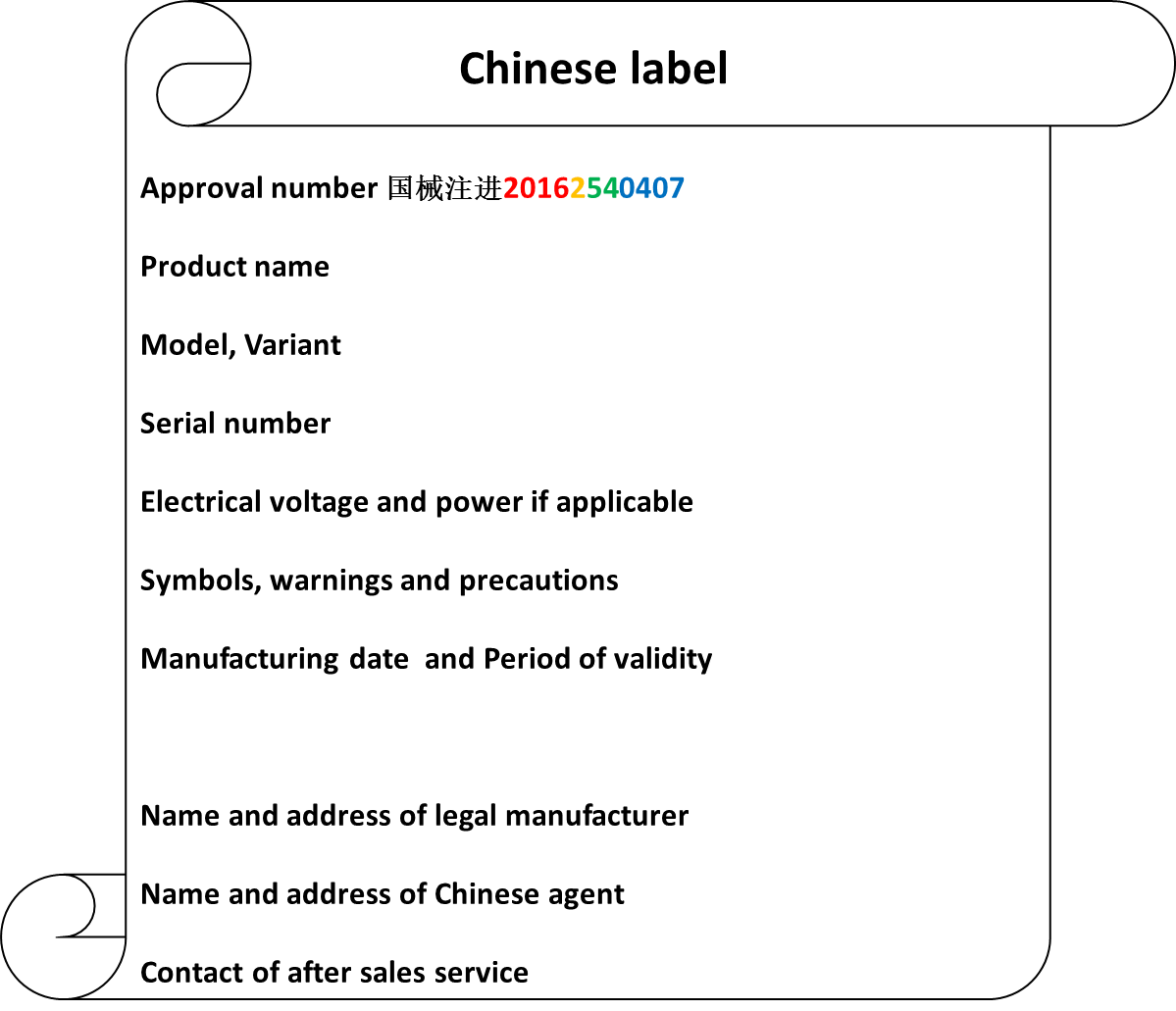
The checklist of IFU is derived from article 11 in guidance of Instructions for use and labels of medical device” (order 6, 2014).
| Requirements on IFU | Consistent with | Comment |
|---|---|---|
| Product name, model, variant | Submission dossier, PTR | ONLY Chinese generic product name |
| Name, address, contact info of Chinese agent and info of after sales service | Submission dossier | After sales service is the main distributor for foreign manufacturer |
| Name, address, contact info of legal manufacturer | Submission dossier | Name in Chinese and English |
| Number of NMPA certificate and product technical requirement (PTR) | NMPA certificate, PTR | Both have the same number |
| Product performance parameters, composition, scope of application | Submission dossier, PTR | Revision after type testing and technical reviews |
| Contraindications, Precautions and warnings | Submission dossier | N.A. |
| Instructions for Installation and for use | Submission dossier | N.A. |
| Product maintenance, storage -and transport condition | Submission dossier | N.A. |
| Manufacturing date and period of validity or expiration date | NMPA certificate, PTR | Period of validity related to validation test of shelf time |
| Accessories list, replacement cycle and - method | Submission dossier, PTR | N.A. |
| Explanation of graphics, symbols and abbreviations | Submission dossier | N.A. |
| Revision date of IFU | Submission dossier | The item should be EMPTY at IFU in Submission dossier. The date can be added after product approval. |
Among label requirements in the table, these items are obligatory in Chinese if limited space on label, otherwise it is to mention in IFU:
See article 13 in guidance of Instructions for use and labels of medical device” (order 6, 2014):
- Product name, model and variant
- Name, address, contact info of legal manufacturer
- Number of NMPA certificate and product technical requirement (PTR)
- Manufacturing date and period of validity or expiration date
Special IFU requirements for electronic active device
There are also Chinese electronic standards series as IEC 60601. Special labelling requirements are to find in newest:
GB 9706.1-2020 (valid as of May, 2026, replacing GB 9706.1-2007)
Corresponding to IEC 60601-1:2012, edition 3, Medical electrical equipment—Part 1: General requirements for safety
YY 9706.102-2021 (valid as of May, 2026, replacing YY 0505-2012)
Corresponding to IEC 60601-1-2:2007, edition 3, Medical electrical equipment-Part 1-2: General requirements for safety-Collateral standards: Electromagnetic compatibility-Requirements and tests
Special IFU Requirements for standalone software
For additional IFU requirements it is wise to check general and product specific guidance. We list just some typical requirements:
Medical device software registration review guidelines
The instructions must reflect the software's functions, usage restrictions, input and output data types, necessary software and hardware, maximum number of concurrencies, interfaces, access control, operating environment (if applicable), performance efficiency (if applicable) and other information, and clarify the software release version……
Software of Artificial Intelligence Assisted Detection of Medical Devices
1. Conclusion of clinical trial
2. Scope of indication
3. Related requirements about data collection device and data collection process
4. If applicable: The software only assists doctors in detecting lesions, and there is the possibility of false negatives/positives. A professional physician should comprehensively give a final conclusion on lesion detection based on the patient’s medical history, symptoms, signs, and other examination results, and verify whether further diagnosis and treatment is needed. decision-making and be responsible for clinical diagnosis results
IFU Requirements for IVD (draft in 2022)
- Product name
- Packing specifications
- Intended use
- Principle of Inspection
- Main components
- Storage conditions and validity period
- Applicable Instruments
- Sample Requirements
- Testing method
- Positive judgment value or reference interval
- Explanation of test results
- Limitations of test methods
- Product Performance Index
- Precautions
- Explanation of label
- References
- Basic Information
- Medical Device Registration Certificate Number/Product Technical Requirement Number
or Medical Device Filing Number/Product Technical Requirement Number
19. Approval Date and Modification Date of IFU
Electronic IFU in China
There is no rule in term of eIFU. In a notification, Chinese authority has said only that eIFU should be consistent with approved IFU.
You might know the European regulation 2021/2226 and 207/2012. In China manufacturer could use eIFU in a wide product group independent of professional user or implant.
We can be your smart database of Chinese IFU. Contact us, we fulfil the highest technical demand plus your customer need with Chinese clients.
Summary
There is a continuous revision of Chinese IFU starting from first version, during product registration to final product approval. To prevent penalty due to non-conformity of Chinese IFU and label by NMPA, a controlling system in term of procedure on Chinese IFU and label in the whole life cycle should be established.
CONTACT US to have a template of requirements on Chinese IFU and label, exclusively in comparison to IFU requirements in article 23, chapter III, annex 1 at MDR.
Do you want to train your marketing colleague working on Chinese labelling without Chinese knowledge, visit our on-demand workshop.