Radar des dispositifs médicaux chinois et mondial
Explore law of drug in CHina. There is supporting guidances. Contact us for your business of drug in China.
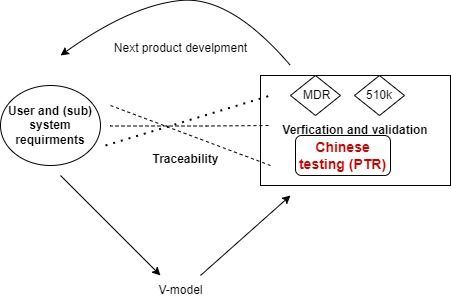
The quality of reply f supplementary questions by Chinese authority matters. It is finding for medical device which manufacturer not only corrects, but also learns.

Learning new country specific regulation in another language is perhaps always a hurdle. Don`t worry. We try to establish a platform where you can learn all regulation by yourself. The registered elearning encloses the video, test, script, meetup with trainer. You will be a giant of regulatory affairs of medical device and In Vitro Diagnostics (IVD) after the course.